Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(2Al+6HCl\rightarrow2AlCl_3+3H_2\left(1\right)\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\left(2\right)\)
\(n_{H_2}=\dfrac{3.785}{24.79}=0.15\left(mol\right)\Rightarrow n_{Al}=\dfrac{2}{3}\cdot0.15=0.1\left(mol\right),n_{HCl\left(1\right)}=0.15\cdot2=0.3\left(mol\right)\)
\(m_{Al}=0.1\cdot27=2.7\left(g\right)\Rightarrow m_{Al_2O_3}=40-2.7=37.3\left(g\right)\Rightarrow n_{Al_2O_3}=\dfrac{37.3}{102}=0.36\left(mol\right)\)
\(\Rightarrow n_{HCl\left(2\right)}=0.36\cdot6=2.16\left(mol\right)\)
\(n_{HCl}=0.3+2.16=2.46\left(mol\right)\)
\(V_{dd_{HCl}}=\dfrac{2.46}{2}=1.23\left(l\right)\)

Sửa đề: 3,785 (l) → 3,7185 (l)
a, \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
b, Ta có: \(n_{H_2}=\dfrac{3,7185}{24,79}=0,15\left(mol\right)\)
Theo PT: \(n_{Al}=\dfrac{2}{3}n_{H_2}=0,1\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,1.27}{40}.100\%=6,75\%\\\%m_{Al_2O_3}=93,25\%\end{matrix}\right.\)
c, \(n_{Al_2O_3}=\dfrac{40.93,25\%}{102}=\dfrac{373}{1020}\left(mol\right)\)
Theo PT: \(n_{HCl}=3n_{Al}+6n_{Al_2O_3}=\dfrac{212}{85}\left(mol\right)\)
\(\Rightarrow V_{HCl}=\dfrac{\dfrac{212}{85}}{2}=\dfrac{106}{85}\left(l\right)\approx1247,06\left(ml\right)\)
d, \(n_{AlCl_3}=n_{Al}+2n_{Al_2O_3}=\dfrac{212}{255}\left(mol\right)\)
\(\Rightarrow m_{AlCl_3}=\dfrac{212}{255}.133,5=\dfrac{9434}{85}\left(g\right)\)
e, \(C_{M_{AlCl_3}}=\dfrac{\dfrac{212}{255}}{\dfrac{106}{85}}=\dfrac{2}{3}\left(M\right)\)

Ta có: \(n_{HCl}=\dfrac{200}{1000}.2=0,4\left(mol\right)\)
\(PTHH:Mg+2HCl--->MgCl_2+H_2\uparrow\left(1\right)\)
a. Theo PT(1): \(n_{Mg}=n_{H_2}=n_{MgCl_2}=\dfrac{1}{2}.n_{HCl}=\dfrac{1}{2}.0,4=0,2\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Mg}=0,2.24=4,8\left(g\right)\\V_{H_2}=0,2.22,4=4,48\left(lít\right)\end{matrix}\right.\)
b. \(PTHH:2NaOH+MgCl_2--->Mg\left(OH\right)_2\downarrow+2NaCl\left(2\right)\)
Ta có: \(n_{NaOH}=\dfrac{\dfrac{20\%.100}{100\%}}{40}=0,5\left(mol\right)\)
Ta thấy: \(\dfrac{0,5}{2}>\dfrac{0,2}{1}\)
Vậy NaOH dư.
Theo PT(2): \(n_{Mg\left(OH\right)_2}=n_{MgCl_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{Mg\left(OH\right)_2}=0,2.58=11,6\left(g\right)\)
a: \(Mg+2HCl\rightarrow MgCl_2+H_2\)
200ml=0,2 lít
\(n_{HCl}=0.2\cdot22.4=4.48\left(mol\right)\)
\(\Leftrightarrow n_{H_2}=2.24\left(mol\right)\)
\(\Leftrightarrow m_{H_2}=n_{H_2}\cdot M=2.24\cdot1=2.24\left(g\right)\)
\(n_{MgCl_2}=2.24\left(mol\right)\)
\(\Leftrightarrow n_{Mg}=2.24\left(mol\right)\)
\(\Leftrightarrow m_{Mg}=2.24\cdot24=53.76\left(g\right)\)

PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
Ta có: \(n_{HCl}=\dfrac{200\cdot71\%}{36,5}=\dfrac{284}{73}\left(mol\right)\)
\(\Rightarrow n_{Fe}=n_{FeCl_2}=n_{H_2}=\dfrac{142}{73}\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Fe}=\dfrac{142}{73}\cdot56\approx108,93\left(g\right)\\m_{FeCl_2}=\dfrac{142}{73}\cdot127\approx247,04\left(g\right)\\m_{H_2}=\dfrac{142}{73}\cdot2\approx3,89\left(g\right)\\V_{H_2}=\dfrac{142}{73}\cdot22,4\approx43,57\left(l\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{Fe}+m_{ddHCl}-m_{H_2}=305,04\left(g\right)\)
\(\Rightarrow C\%_{FeCl_2}=\dfrac{247,04}{305,04}\cdot100\%\approx80,99\%\)
Fe + 2HCl ➝ FeCl2 + H2
mHCl = 200.71% = 142 (g) => nHCl = \(\dfrac{284}{73}\) (mol)
nFe = \(\dfrac{1}{2}\) nHCl = \(\dfrac{142}{73}\) (mol) => m ≃ 108,9 (g)
nH2 = nFe => V ≃ 43,57 (l)
nFeCl2 = nFe => C% ≃ 80%
(Mk nghĩ bạn nên kiểm tra lại đề vì số liệu không được đẹp cho lắm)

a)
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: Mg + 2HCl --> MgCl2 + H2
_____0,1<---0,2<-------0,1<---0,1
=> mHCl = 0,2.36,5 = 7,3 (g)
=> \(m_{ddHCl}=\dfrac{7,3.100}{7,3}=100\left(g\right)\)
mdd sau pư = 0,1.24 + 100 - 0,1.2 = 102,2 (g)
\(C\%\left(MgCl_2\right)=\dfrac{0,1.95}{102,2}.100\%=9,2955\%\)
b)
CTHH: AaOb
PTHH: \(A_aO_b+2bHCl->aACl_{\dfrac{2b}{a}}+bH_2O\)
____________0,2------->\(\dfrac{0,1a}{b}\)
=> \(\dfrac{0,1a}{b}\left(M_A+35,5.\dfrac{2b}{a}\right)=13,5\)
=> \(M_A=\dfrac{64b}{a}=\dfrac{2b}{a}.32\)
Nếu \(\dfrac{2b}{a}=1\) => MA = 32 (L)
Nếu \(\dfrac{2b}{a}=2\) => MA = 64(Cu)

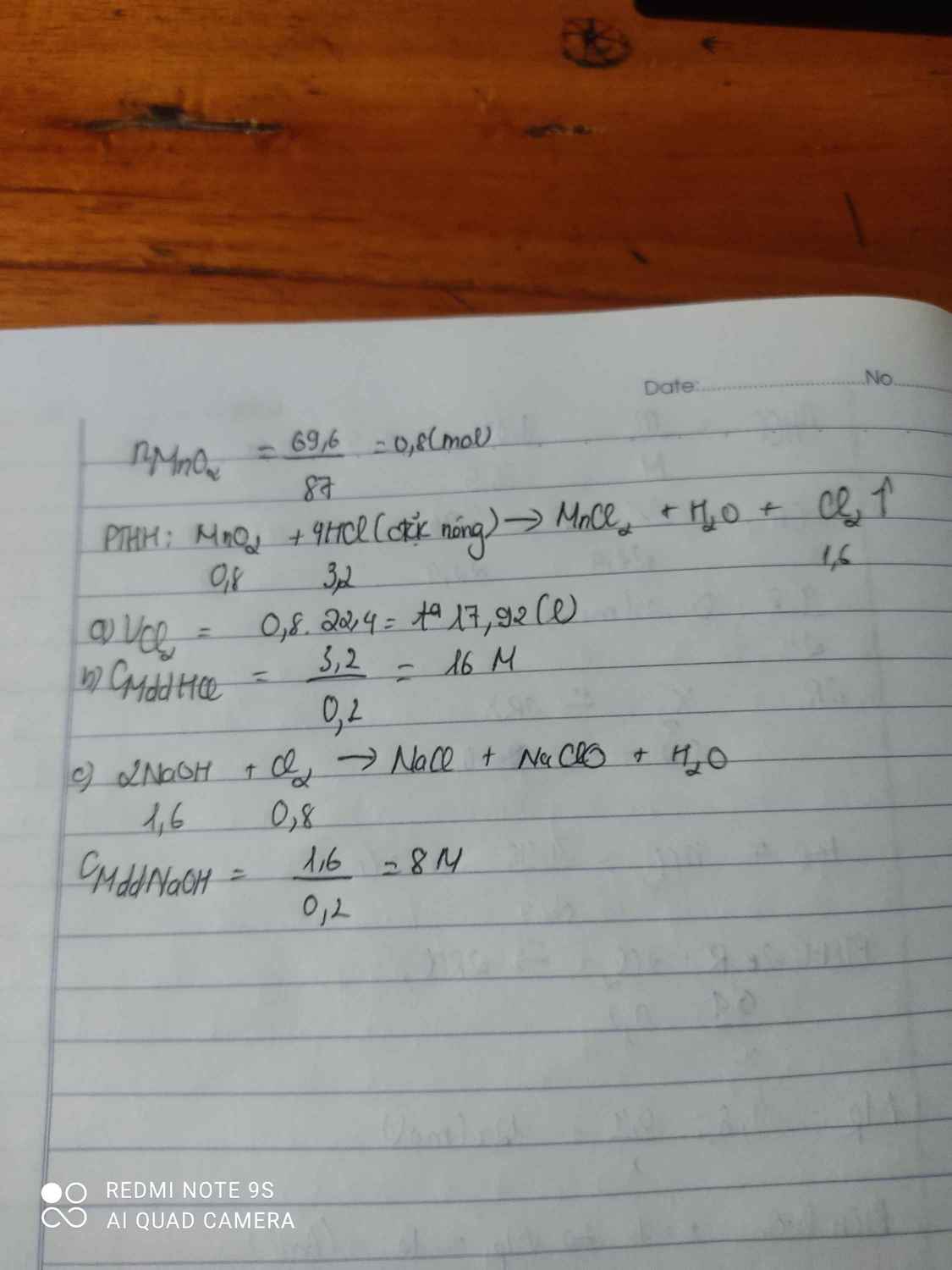
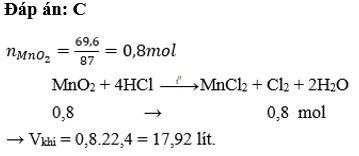

PTHH: \(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+2H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{MnO_2}=\dfrac{17,4}{87}=0,2\left(mol\right)\\n_{HCl}=0,5\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,2}{1}>\dfrac{0,5}{4}\) \(\Rightarrow\) MnO2 còn dư, tính theo HCl
\(\Rightarrow n_{Cl_2}=0,125\left(mol\right)\) \(\Rightarrow V_{Cl_2}=0,125\cdot22,4=2,8\left(l\right)\)